
The Italian town of Larderello was created to exploit the mineral riches of boraciferous fumaroles. Its chemical production was soon eclipsed by geothermal power generation. The minerals found around the area (33 in total) include six rare borates, which were first described there - the type locality.
In 1827 an engineer’s mind was applied to exploiting “Valle del Diavolo” - Devil’s Valley, an area in southern Tuscany, Italy. The man was Count de Larderel, Boron Magnate & Father of Geothermal Power. He’s shown above, with the mineral which carries his name, but is named after his grandson.
The region has a history of phreatic volcanic eruptions, with the steam explosions creating a dozen explosion craters 30–250 m in diameter. The last large eruption was in 1282, forming the Lago Vecchienna crater, now Boracifero Lake.
Larderellite is unusual chemically, having both ammonium and borate ions; ammonium borate hydroxide. It’s fumarolic mineral, forming around the neck of fissures which issue steam at high temperatures and pressure. These “soffioni boraciferi” can be quite violent, with water vapour venting at 20 bar and 230°C, ejecting dirt and gravel at considerable velocity. Some idea of the area can be gleaned from the experiences of Targioni Tozzetti, an Italian naturalist, who visited in 1742 -
“His attention, however, was soon attracted to the scene around him; he stood close to a yawning gulf, from which issued rumbling noises and disagreeable odours; he wished to look down and peep into the mysterious chasm to learn something of its nature, but his temerity was rewarded by a surly growl from within, and his guide told him that the noise sometimes resembled a hundred bellows, as if Vulcan himself were hard at work, while flames issued forth at night after very hot days. “Though he saw no fire, the vapour served as a warning to him to keep at a considerable distance; but before long he came upon more vapour vents, soffioni, and little lagoni, or ponds of muddy blue waters, boiling vehemently, the imprisoned gases producing bubbles increasing in size till sufficiently large to cause them to burst. Dense white vapour, smelling strongly of rotten eggs, rose from the lagoni and ascended to a considerable height into the atmosphere. The ground on which he stood was soft and crumbled under his feet; the decomposed rocks and some of the efflorescent minerals were new to him, and the subject of many curious speculations.
 Some of Tozzetti’s curious efflorescent minerals
Some of Tozzetti’s curious efflorescent minerals Sborgite, Sassolite, & Larderellite
Sborgite, Sassolite, & Larderellite
“....The whole of the valley was apparently studded with such lagoons, an attempt to define the number of which was futile, connected as they were in many places by cross fissures and superficial cracks. Not a tree was visible throughout the extent of the valley; the opening of a new fissure was the signal for the destruction of all neighbouring shrubs, scorched by the subterranean heat. Should any luckless wight approach a lagoon too closely, he would stand the chance of sinking into a quagmire, or losing a leg. Sheep occasionally fell victims when rushing too carelessly along and after remaining a short time in the water, nothing but a bleached skeleton remained. Though this picture is perhaps rather overdrawn, the temperature being very considerably above the boiling point of pure water, very serious and generally fatal accidents must have resulted. It would be untrue to say that the soffioni were utterly useless. The skilled peasants would cleverly manage to roast their chestnuts in sacks placed over these vapour vents….”
In 1777 The Grand Duke of Tuscany’s chemist, M. Hoeffer, analysed the encrustations and dissolved chemicals in the soffioni and lagoni. He found they contained appreciable quantities of boron chemicals - up to nine percent boracic acid from one lagone. Boracic acid was in demand in Europe for the glass industry. The peasants of the area would soon have work in the Valle del Diavolo. The Devil’s Soffioni had much more utility than just roasting nuts. In 1818 white glass was exhibited in Florence, prepared with borax made from the lagoons.
Industrial exploitation had started. As is above, the following is from The Mineral Resources of the Central Italy, by William Paget Jervis, 1862 -
“Messrs. Gazzeri and Brouzet worked the lagoons of Monte Rotondo from 1815 to 1818, employing as their engineer Sig. Ciaschi, who made further improvements by constructing artificial lagoons round the dry soffioni, to utilize the hitherto waste vapours. The poor fellow was one day superintending an operation of this nature, in 1816, when he fell into a fissure: he was dragged out half dead, and only lingered for a few days, during which time he suffered the most excruciating torture from violent spasms and frightful burns. Gazzeri and Brouzet with great difficulty managed to export to France 3 tons 5 ½ cwt. of very impure crude boracic acid in the nine and a half months ending April 1, 1818.”
The fumaroles’ gaseous eructations are far from rich in boron. The gas is predominantly superheated steam, containing only 0.03% boric acid (a more modern term for boracic acid) -
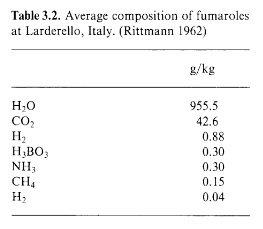
 At the mouth of a dry soffione, the boric acid sublimes out of the live steam and the mineral sassolite is deposited as an encrustation. Initially the sassolite is in the form of delicate hexagonal plates which have crystallised directly from the gaseous phase. An example of this “sublimation sassolite” is shown below (from a different locality) -
At the mouth of a dry soffione, the boric acid sublimes out of the live steam and the mineral sassolite is deposited as an encrustation. Initially the sassolite is in the form of delicate hexagonal plates which have crystallised directly from the gaseous phase. An example of this “sublimation sassolite” is shown below (from a different locality) -

 Sublimated Sassolite
Sublimated Sassolite
La Fossa Crater, Vulcano Island
Unless preserved by a field collector, this mineral form is only of a temporary nature. Rain dissolves the sassolite, which can then re-crystallise into crusts around pools. These secondary deposits are usually an admixture with other sublimated minerals washed from the fumarole, such as sulphur -
 Sassolite encrustation with sulphur
Sassolite encrustation with sulphur
Larderello
At a dry soffione, very little of the boric acid in the superheated steam actually crystallises out as sassolite. Most is lost to the atmosphere. At a lagone, where a pool of water covers a fumarole, things are a lot different. The live steam bubbles up through the pool, and the majority of the boric acid is dissolved in the water. The pool becomes steadily more enriched in dissolved boric acid. Sassolite encrustations can occur at these lagoni, and what Gazzeri and Brouzet had attempted was to harvest the solution.
They used wood and charcoal to boil down waters from existing lagoni, and artificial lagoons constructed by poor Sig. Ciaschi. Although the result of their labours sold well to the French glass-making industry, fuel became scarce and the economics of further exploitation was in doubt, especially since the enterprise had cost a life.
The mind of an engineer, François de Larderel, elegantly solved the problems.
Water was in short supply to create new lagoni, therefore de Larderel had them enclosed for condensation, at the same time scoring massively in terms of health and safety -

 Lagone Coperto
Lagone Coperto
In order to concentrate the solution of boric acid, he cascaded the water from one lagone to the next -
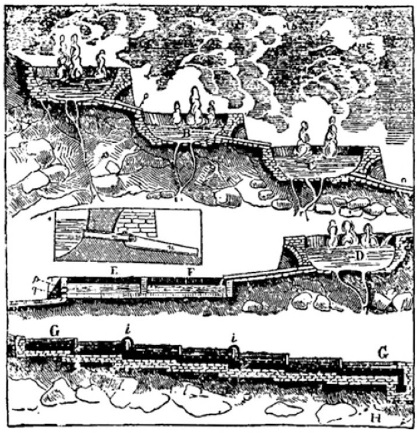 Lagoni a cascata
Lagoni a cascata
Next de Larderel solved the fuel problem. In order to boil down the solutions obtained from lagoni, Gazzeri and Brouzet had denuded the surrounding countryside of timber for burning. Instead of boiling the fluid, de Larderel commissioned evaporation tanks that were heated by the steam contained by, and led from, the lagoni coperto (the brick hemispherical domes he built over the lagoni). Thus the whole enterprise was operated by geothermal energy, the first time such a power source was used in industry.
He began to produce large quantities of boric acid, and soon began drilling down to open up new soffioni. The family business was established. Some of the boreholes produced large quantities of very high pressure steam, and naturally he (and his offspring who continued the enterprise) thought of utilising this excess power. By 1870 pumping and drilling at Larderello was being accomplished using geothermal power. It was not until 1904 when the world’s first geothermal electricity was generated; in the following photo Prince Ginori Conti (who’d married into the de Larderel family) observes the pressure of a closed borehole (5 bar) before opening up the steam engine. The engine drove a small dynamo which lit a series of incandescent lamps.
 First Geothermal Electricity Generation, April 24, 1904, Larderello, Italy
First Geothermal Electricity Generation, April 24, 1904, Larderello, Italy
 Larderello in 1950, already dominated by the geothermal power station.
Larderello in 1950, already dominated by the geothermal power station.

Larderello now produces 10% of the world's entire supply of geothermal electricity, amounting to 4,800 GWh per year and powering about a million Italian households.
Returning to the minerals of Larderello, volcanic sublimates can still be found there. Not all the steam is being harnessed by the power station. The countryside is still dotted with areas of fumarolic activity. Soffioni can be seen in the following videos -
Some of the rarer minerals found at the Larderello soffioni and lagoni include -
- Sassolite
- Larderellite (TL)
- Biringuccite (TL)
- Ammonioborite (TL)
- Sborgite (TL)
- Santite (TL)
- Nasinite (TL)
Anyone visiting the area to explore the fumaroles and lagoons should keep in mind that some existing natural lagoni in the Devil’s Valley are as dangerous now, as they were in the 1800s -
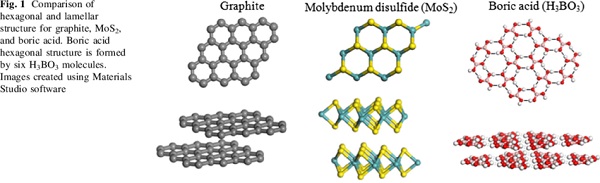
Postscript on Sassolite. A distinctive characteristic of the mineral is it’s greasy feel. This is because boric acid is one of a specialized class of materials known as lamellar solids. The basic molecule H3BO3 -
When crystallised, the boric acid molecules arrange as layers. Within each layer boric acid molecules are held together by strong hydrogen bonds. The layers themselves are held together by weak van der waals interactions. This means the layers can slide over each other easily; so giving boric acid a solid lubricating property, like graphite or molybdenum disulphide. It’s used by carom players to lubricate their boards, and (unrelated to being a lamellar solid) gives dogs testicular atrophy.
References :
On the Boracic Acid Lagoons of Tuscany, John Bowring, 1839
Excursion to Pomarance and the Boracic Acid Lagoni, O. Blewitt, 1874
Boracic Acid, The Mineral Resources of the Central Italy, W.P.Jervis, 1862
Geothermal Power Generation: Developments and Innovation, Ron DiPippo
Hydrothermal Mineral Deposits: Principles and Fundamental Concepts, Franco Pirajno
Borax, Boric Acid, and Boron-From Exotic to Commodity, Jaime Wisniak
Toxicologic studies on borax and boric acid, Weir & Fisher, 1972
And Now for Something Completely Different
____________________